Spins in Organic Radicals
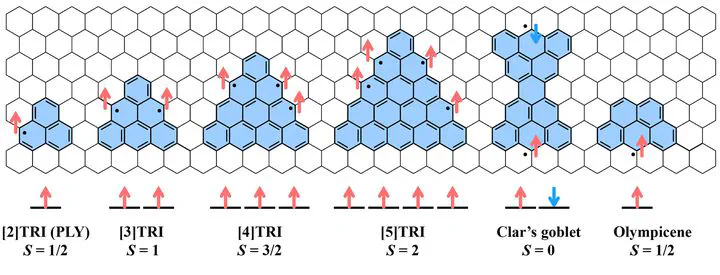
In contrast to most closed-shell organic systems, radical molecules feature unpaired electrons, leading to fascinating open-shell electronic structures. Our research focuses on polycyclic aromatic hydrocarbons (PAHs) with precisely engineered topologies that stabilize radical states. By designing specific molecular shapes—such as triangular graphene fragments—and incorporating unique geometries like Clar’s goblet and olympicene, as well as introducing heteroatoms, we are able to control their spin states (e.g., S = 1/2, 1, 3/2, etc.). These radicals act as fundamental building blocks for metal-free materials with extended π-conjugation, providing a powerful platform to explore electron correlation and magnetism within carbon-based frameworks.